Story highlights
Ovarian cancer is one of the most lethal types of cancer
Men who are carriers of the gene have an increased risk of other types of cancer
A previously unrecognized link has been found between ovarian cancer and a gene on the X chromosome, according to a new study.
The finding, which reveals that a father’s genes play an important role in a woman’s ovarian cancer risk, could change the way doctors look for and treat one of the most lethal types of cancer.
The gene, called MAGEC3, is still under investigation by scientists. A normal version is thought to be protective against tumor formation, according to the study, which was published last week in the journal PLOS Genetics. However, mutations may result in the unrestrained growth and reproduction of cells, leading to cancer.
Ovarian cancer has a five-year survival rate of approximately 45%, according to the American Cancer Society. In 2015, there were about 1.5 million cases of ovarian cancer worldwide and more than 161,000 deaths, making it the eighth most common cause of death from cancer.
“Ovarian cancer is sometimes called the silent killer. It’s hard to detect, and it’s hard to predict,” said Kevin Eng, an associate professor of oncology at the Roswell Park Comprehensive Cancer Center and a leading author of the study.
The study identified the gene by comparing more than 3,000 grandmother/granddaughter pairs from the Familial Ovarian Cancer Registry at the Roswell Park Cancer Institute in Buffalo, New York.
Founded in 1981, the registry contains more than 50,000 participants from more than 2,600 families with a history of ovarian cancer.
“Our study really leveraged this large familial registry that we’ve had running at Roswell Park for over 35 years,” Eng said. “The familial cancer registry is, I believe, the oldest ovarian cancer registry in the world.”
Eng and his colleagues theorized that, because women have two X chromosomes but men have only one, a mutated gene on the X chromosome would be shared twice as often between paternal grandmother/granddaughter pairs than maternal pairs.
The authors of the study “really thought outside the box,” according to Dr. Krishnansu Tewari, a professor of obstetrics and gynecology and interim director of the Division of Gynecologic Oncology at the University of California, Irvine, who was not involved in the research.
“If the problem is on the X (chromosome) and the sisters are all affected but the mom isn’t, that means it’s coming from the dad, and originally it came from an affected grandmother on the paternal side,” Tewari said.
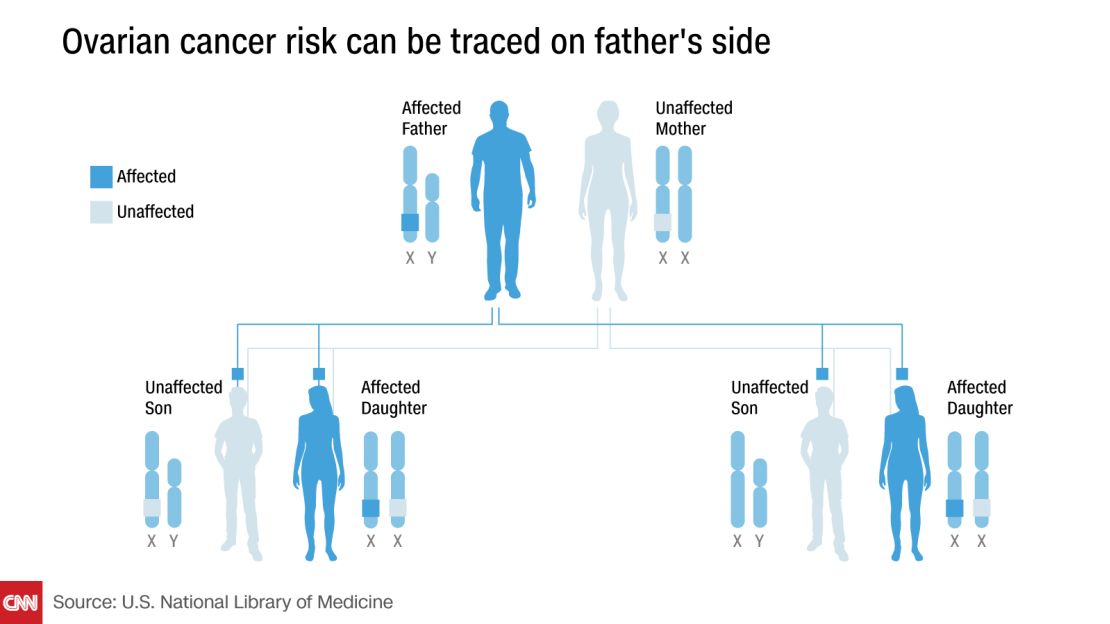
The researchers found that women whose paternal grandmothers had ovarian cancer were twice as likely to develop ovarian cancer themselves, compared with those whose maternal grandmothers had ovarian cancer – consistent with the theory that the responsible gene was on the X chromosome.
Overall, 28.4% of the granddaughters in the paternal pairs also developed ovarian cancer, compared with 13.9% of the granddaughters in the maternal pairs.
Notoriously hard to diagnose
The results of the new study may provide valuable insight into one of the most difficult cancers to identify and diagnose at a treatable stage, according to Tewari.
“Ovarian cancer doesn’t have any specific early symptoms,” he said. “It’s the most lethal gynecologic cancer because there’s a paucity of early symptoms and absence of a validated screening test.”
Screening tests include vaginal ultrasound or a blood test that looks for CA-125, a protein that is often elevated in patients with ovarian cancer. However, these tests typically detect only advanced cases and have a high false positive rate, meaning many people with a positive test do not actually have cancer.
“When symptoms manifest, they are often indicative of advanced disease: bloating, abdominal and pelvic discomfort, sometimes pelvic pain, difficulty with the bowels, pressure on the bladder,” Tewari said.
“All those symptoms are nonspecific symptoms that really reflect advanced disease.”
Due to the advanced nature of most ovarian cancer cases, treatment typically includes aggressive surgery and heavy doses of chemotherapy, according to Tewari.
“The cornerstone of treatment is aggressive cytoreductive surgery – what we call surgical debulking, where you cut out all the tumor in order to leave the patient with as little residual disease as possible,” he said. “This sets the stage for chemotherapy.
“That said, the 10-year disease-free survival is still under 10%,” he added. “That is why it is so important for patients to voluntarily enroll into clinical trials that are testing new, promising drugs.”
Looking beyond first-degree relatives
The study’s findings could change the way doctors perform family histories for gynecologic cancers, according to Tewari.
“I think it’s definitely going to have an immediate impact on how we take family histories and how much importance we place on second-degree relatives such as a paternal grandmother,” he said.
“Whenever we talk about genetic testing, we’re usually so focused on the first-degree relatives – the mother, the daughter, the sister – and we always think of the grandmother as a second-degree relative,” Tewari added. “But in this case, a paternal grandmother could be very critical.”
Before this study, the BRCA1 and BRCA2 genes were the ones most commonly associated with ovarian cancer, accounting for around 15% of all ovarian cancer cases, according to Tewari. However, these genes are located on autosomes – i.e. not the X or Y chromosome – and follow a different inheritance pattern than genes on the X chromosome.
“If a father has a mutation on chromosome 3 and the mother doesn’t, there’s only a 50% chance for each child to inherit a bad chromosome 3,” Tewari continued. “But if the father’s one X chromosome has a mutation, then all of his daughters will inherit that bad X chromosome because the father determines the sex of the child.”
Eng said that “the weird thing about this pattern is also that Dad will always pass on the same X chromosome to all of his daughters. So unlike BRCA, you have this all-or-nothing pattern, so you and every one of your sisters is going to have the same X chromosome from dad.”
Individuals with the mutated gene on the X chromosome also developed ovarian cancer at a surprisingly young age, according to Eng.
“The median age of onset for ovarian cancer is 65, but for the early-onset ones, we’re talking about women getting cancer earlier than 45,” he said.
Men who carry the gene may still be at risk
Men who carried the mutated gene were also not in the clear, the researchers found. Though men cannot develop ovarian cancer, those with the mutation were significantly more likely to develop other types of cancer, particularly prostate cancer, according to Eng.
“To some extent, we did see a bump in prostate cancers for the intervening dad,” Eng said. “This suggests that there could be other cancers that are caused by this particular gene.”
Join the conversation
What this means for the future diagnosis and treatment of ovarian cancer is still unclear, Tewari says.
“I think that part of the study is very preliminary at this point,” he said.
“Once the putative gene is validated, we can sequence it, we can clone it, we can test for it, and we can potentially save many women from ovarian cancer and some men from prostate cancer,” Tewari added. “The exact number of lives saved will really depend on what percentage of presumably sporadic cases of ovarian cancer are indeed X-linked.”
According to Eng, knowing your family history is still among the best ways to identify your risk for ovarian cancer. “One of the best preventative measures is talking with your family, knowing if you have a family history of ovarian cancer and communicating that to your primary care physician,” he said.