Story highlights
A new study says the benefits of the world's first malaria vaccine weaken over years
Another trial is looking at expanded dosage of Mosquirix
The United States eradicated malariaby spraying mosquito breeding grounds with DDT in 1951, roughly two decades after most of Western Europe eliminated the disease.
History then offers hope, even as the world’s first malaria vaccine, called RTS,S or Mosquirix, faces challenges: The effectiveness of the shot fades over time, according to a study released Wednesday.
The vaccine’s waning effect occurred especially among children living in areas with high rates of the disease.
“Overall our study shows that RTS,S can benefit children, but suggests that a fourth dose may be important for sustaining this protection over the long term,” Dr. Ally Olotu, lead author of the research published in the New England Journal of Medicine, said in a news release.
“This publication is important because it’s the longest follow-up we’ve seen so far, and it confirms what we already know,” said Ripley Ballou, head of the GlaxoSmithKline global vaccine U.S. research and development center.
Ballou worked on the vaccine, which was developed by GlaxoSmithKline in collaboration with the PATH Malaria Vaccine Initiative and funded in part by the Bill and Melinda Gates Foundation. The scientists created RTS,S especially for infants and young children living in malaria-endemic regions in sub-Saharan Africa.
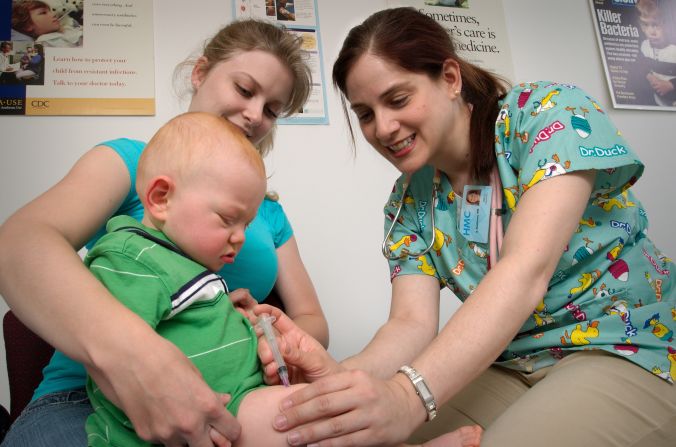
Mosquirix (PDF), which is injected into a baby’s thigh muscle, is intended to protect against malaria caused by the Plasmodium falciparum parasite.
Vulnerable children
Malaria can cause high fever, chills, flu-like symptoms and severe anemia, symptoms that are dangerous for pregnant women and children. The World Health Organization estimates that 214 million new cases of malaria and 438,000 malaria deaths occurred worldwide in 2015, with the African region accounting for most. Children under age 5 are particularly susceptible to infection and death; 306,000 malaria deaths were recorded in this age group in 2015.
With these dying babies in mind, the European Medicines Agency gave Mosquirix the thumbs-up just last year.
In approving the vaccine, the agency looked at evidence derived from a large clinical trial conducted in seven African countries. The agency considered the safety profile of Mosquirix acceptable, even though data showed that it provided only modest protection against the most dangerous form of malaria in children.
Specifically, in the 12 months after vaccination, the vaccine was effective at preventing a first or only clinical malaria episode in 56% of children between the ages of 5 months and 17 months and in 31% of children between the ages of 6 weeks and 12 weeks.
Although its effectiveness was limited, the European Medicines Agency concluded that the vaccine could still help children living in areas where deaths from malaria are frequent.
Fewer cases of severe malaria
The newly published research is based on a seven-year study of 447 children in Kenya. When they were between 5 months and 17 months old, the children received either three doses of RTS,S or a control vaccine. Of all the children, 312 remained in this small study for the complete follow-up period.
The researchers found that the risk of getting malaria among children vaccinated with Mosquirix was 35.9% less than in the control group during the first year. However, this protection fell to 4.4% after seven years. In fact, slightly more cases of the disease occurred among vaccinated children living in areas with high rates of malaria than in the control group five years after vaccination.
“While our results raise the possibility that being exposed to very high levels of malaria parasites may undo some of the benefits of RTS,S, our sample size was too small to draw any definitive conclusions about the long-term efficacy of the vaccine,” said Dr. Philip Bejon, senior author of the study and a professor of tropical medicine at Oxford University.
Ballou explained that the newly published study is actually an extension of one arm of the original phase 2 clinical trial conducted by GlaxoSmithKline. (A phase 2 trial is a safety test in which the drug is given to humans.) This new study followed the children for a longer time.
In fact, the dwindling benefits of Mosquirix – first seen in the shorter time frame of the original study – motivated the scientists to change the dosage, adding a shot 18 months after the third.
Today, the possible benefits of a fourth shot of Mosquirix are being studied in an ongoing clinical trial. GSK’s pricing model covers only the cost of manufacturing the vaccine, with small returns reinvested in research.
Despite the shot’s less-than-perfect protection against disease, Ballou noted, “What I’m reassured about is, there were fewer cases of severe malaria.”
As for why it is so difficult to eradicate malaria in Africa when it’s been done in other areas of the world, Ballou said, “It definitely has to do with the mosquito.” The species of mosquito found in Africa not only get infected at higher rates than those in America, they’re better at transmitting the infection to humans.
Join the conversation
Also, scientists were able to aggressively eliminate breeding sites in America, essentially, destroy the mosquitoes where they live. By contrast, in Africa, mosquitoes breed in areas that are much more difficult to control, so the same methods simply don’t work.
That’s why all possible approaches to malaria prevention – including bed nets, indoor spraying and the vaccine – are so important, said Ballou. “It’s a terrible illness.”